What Are The Characteristics Of Covalent Bonds
These are mostly gaseous and even a slight negative or positive charge at opposite ends of a covalent bond gives them. Characteristics of the bonds.

Single Covalent Bond Definition And Examples
The most notable exception to this octet is hydrogen needing only two electrons.

What are the characteristics of covalent bonds. The molecules that make up living things are covalently bonded for example and covalent bonds are more common in nature than ionic bonds overall. Ionic bonds result from a transfer of electrons from one species usually a metal to another usually a nonmetal or polyatomic ion. Covalent bonds have a definite and predictable shape and have low melting and boiling points.
Activate the protein by changing its geometric shape. In Polar Covalent chemical bonding electrons are shared unequally since the more electronegative atom pulls the electron pair closer to itself and away from the less electronegative atom. For example in a carbonyl group between a carbon atom and an oxygen atom.
A bond in which a pair or pairs of electrons is shared by two atoms. In some cases the bonds are rather loose and both come together only during the course of a reaction. When elements combine there are two types of bonds that may form between them.
Covalent bonds can be either be Polar or Non-Polar in nature. Covalent ionic and metallic. Polymers are held together by primary bonds covalent bonds and secondary bonds van der Waals and hydrogen bonds.
Covalent bonds result from a sharing of electrons by two or more atoms usually nonmetals. In other cases they are firmly bound together by covalent bonds. In chemistry a double bond is a covalent bond between two atoms involving four bonding electrons as opposed to two in a single bondDouble bonds occur most commonly between two carbon atoms for example in alkenesMany double bonds exist between two different elements.
The activating role of a cofactor is to either. Molecular compounds refer to covalently-bonded species generally of low molecular mass. In covalent bonding there is a sharing of valence electrons the s and p shells to complete an octet a group of eight electrons around atoms.
Intramolecular bonds are the bonds that hold atoms to atoms and make compounds. Due to the difference in bonding styles covalent bonds can form between atoms of the same element such as hydrogen gas which has the formula H 2 but ionic bonds cant. Lewis theory Gilbert Newton Lewis 1875-1946 focuses on the valence electrons since.
There are 3 types of intramolecular bonds. Other common double bonds are found in azo. They can be easily broken into its primary structure as the atoms are close by to share the electrons.
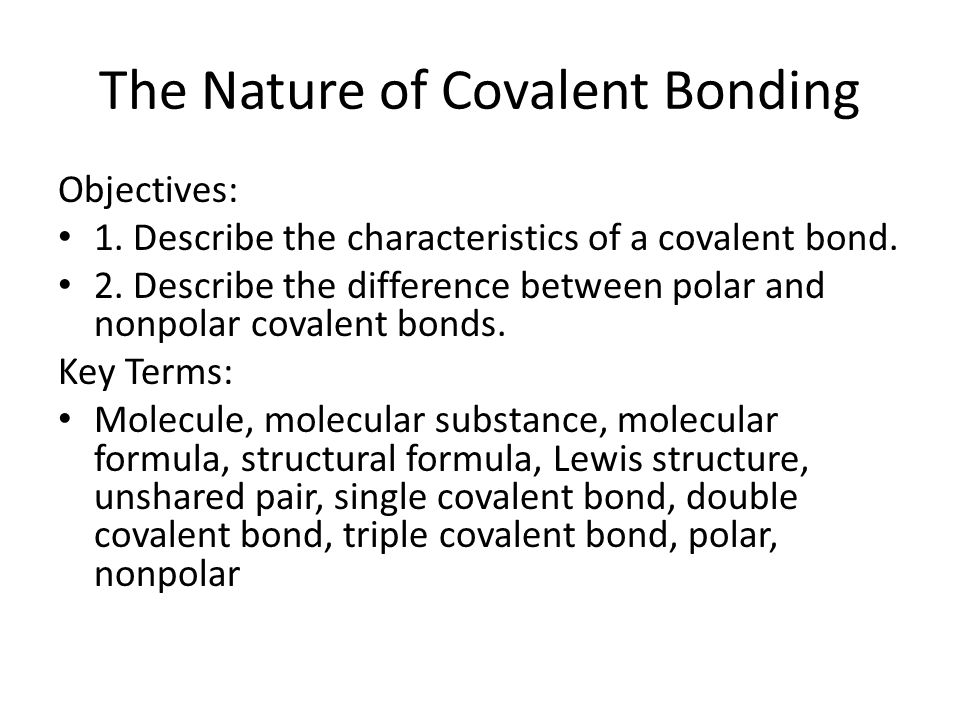
The Nature Of Covalent Bonding Objectives 1 Describe The Characteristics Of A Covalent Bond 2 Describe The Difference Between Polar And Nonpolar Covalent Ppt Download

Covalent Bond Definition Properties Examples Facts Britannica

Properties Of Ionic And Covalent Compounds Ppt Download

Ionic Bonds Vs Covalent Bonds Chemtalk

Bonding And Reactions Whs Chemistry I

Covalent Bond Definition Types And Examples
What Does A Covalent Bond Mean Quora

Characteristics Of Covalent Compounds Chemical Bonding 16h Youtube

Characteristics Of Covalent Compounds Youtube

What Are Covalent Compounds Why Are They Different From Ionic Compounds List Their Characteristic Properties Sarthaks Econnect Largest Online Education Community
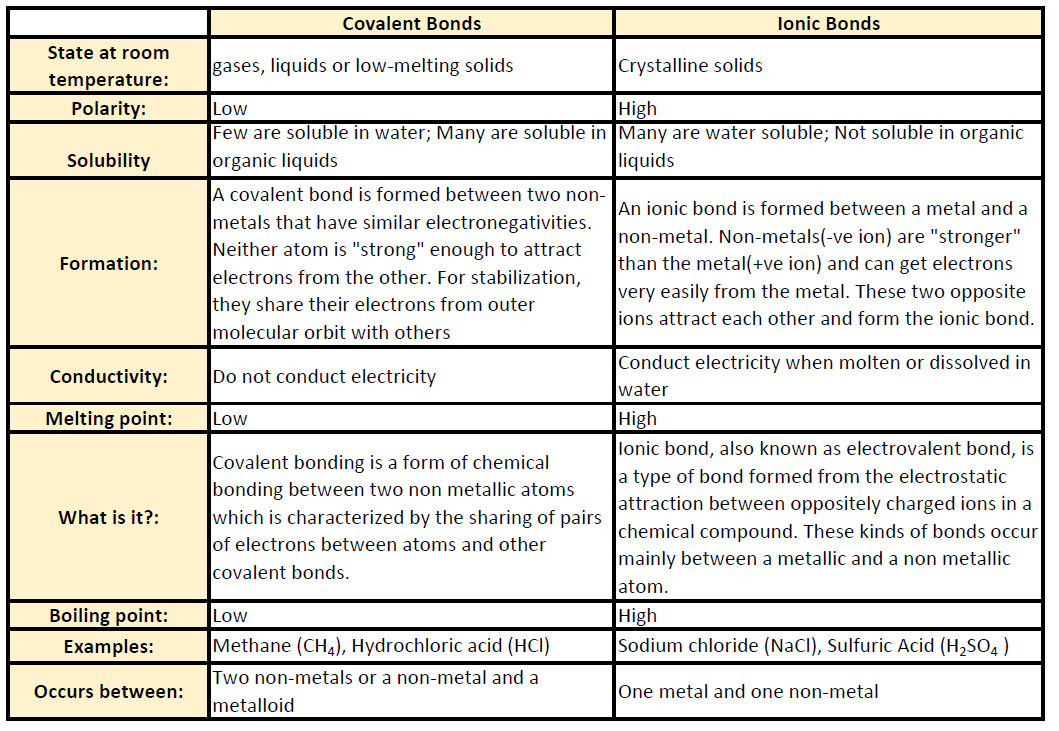
Ch150 Chapter 4 Covalent Bonds And Molecular Compounds Chemistry

Covalent Bond Definition Types And Examples
Difference Between Covalent And Coordinate Bond Definition Formation Examples Comparison
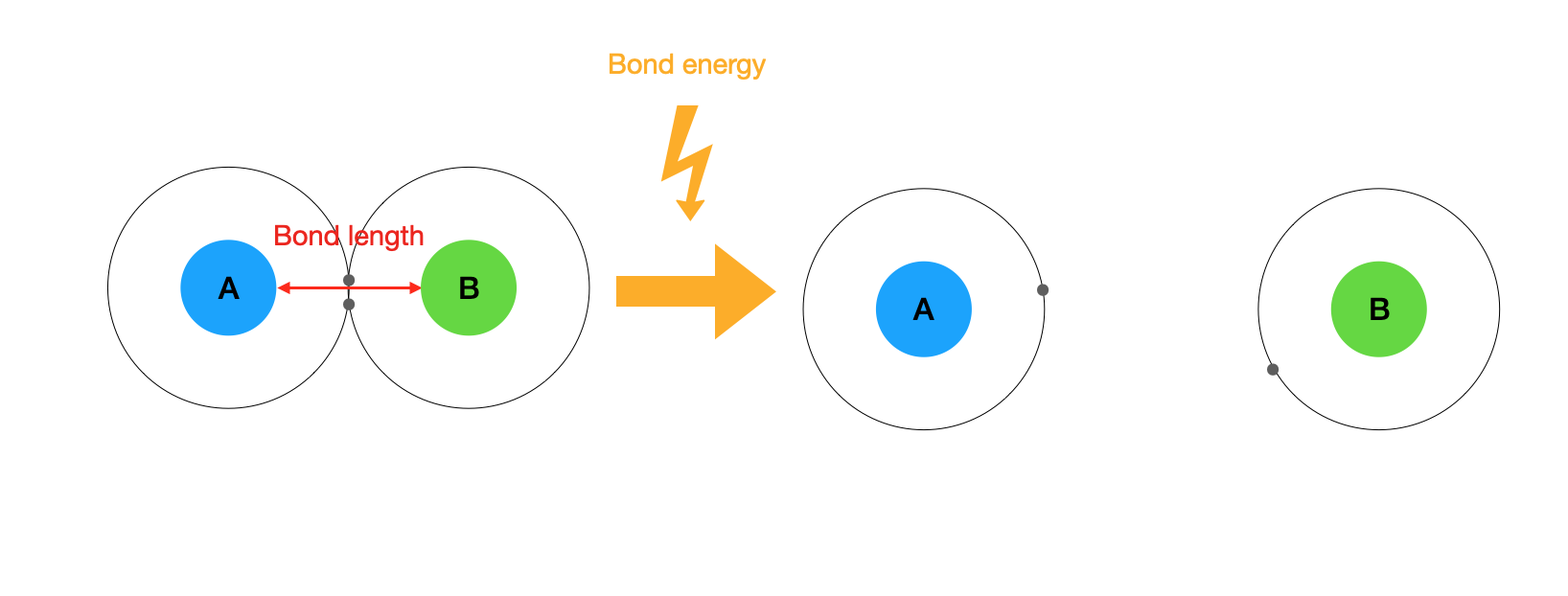
5 8 Characteristics Of Covalent Compounds Chemistry Libretexts

Covalent Bond Biology Dictionary

Covalent Bonding Objectives 1 Describe The Characteristics Of A Covalent Bond 2 Describe The Difference Between Polar And Nonpolar Covalent Bonds Ppt Download
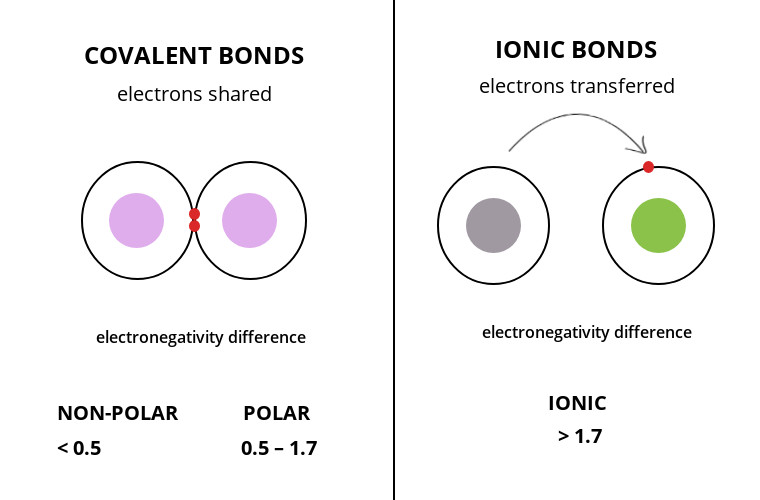
15 Major Difference Between Covalent And Ionic Bonds With Table Core Differences
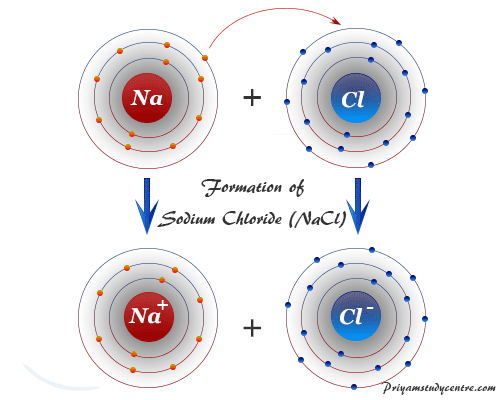
Covalent Bond Types Definition Properties Examples

Covalent Bond Examples Formation Properties What Is A Covalent Bond Video Lesson Transcript Study Com