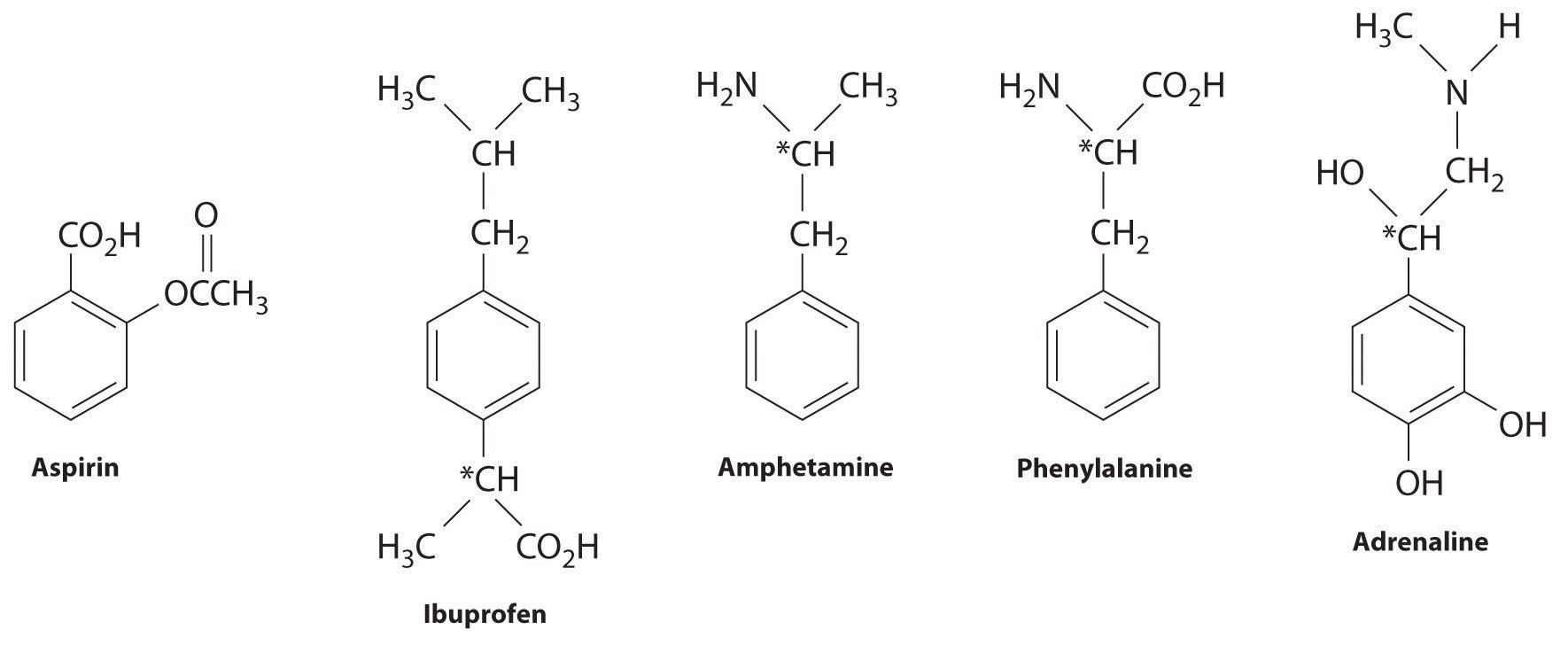
What Are The Difference Of The General Classes Of Organic Compounds
This is of course easiest to do when an atom bears a charge of 1 in the first place and becomes progressively more difficult as the overall charge becomes negative. The simplest ketone is acetone R R methyl with the formula CH 3 COCH 3Many ketones are of great importance in biology and in industry.
Classes Of Organic Compounds Boundless Chemistry
IUPAC Nomenclature Of Organic Compounds - Nomenclature is the uniform system for naming the compounds.
What are the difference of the general classes of organic compounds. Inorganic compound is a chemical compound which is the opposite of an organic compound. Factor 1 Charge. Visit BYJUS to learn more about it.
Ozone O 3 Is A Powerful Oxidant For Cleaving Alkenes To Carbonyl Compounds. Atom cluster compounds including metal clusters have been unwittingly used by humans since antiquity. Its a powerful oxidant and since its discovery in the mid 1800s by Schönbein has found use in the cleavage of carbon-carbon multiple.
Main Difference Addition vs Substitution Reactions. Most chemical synthesis and identifications are based on these reactions. Know IUPAC system rules and how to name organic compounds Types of Chemical Nomenclature Compositional Substitutive.
Molecules of organic compounds except that of hydrocarbons can be divided into two parts a reactive part which is referred to as functional group and a skeleton of carbon atoms called alkyl group. They constitute the framework on which functional groups are located in other classes of compounds and provide an ideal starting point for studying and naming organic compounds. Addition reactions substitution reactions and elimination reactions are fundamental reactions in organic chemistry.
As mentioned on one Reagent Friday back in the day ozone does more than absorb UV radiation in the upper atmosphere and cause breathing problems in traffic-clogged cities. Ethers are compounds having two alkyl or aryl groups bonded to an oxygen atom as in the formula R 1 OR 2The ether functional group does not have a characteristic IUPAC nomenclature suffix so it is necessary to designate it as a substituent. For detecting nitrogen sulphur and halogens we can use the sodium fusion test Lassaignes test.
Removal of a proton H decreases the formal charge on an atom or molecule by one unit. This difference suggests such compounds may have a triple bond two double bonds a ring plus a double bond or two rings. Examples include many sugars.
Tests for the Functional Groups Present in Organic Compounds. It can be considered as a compound which does not contain carbon to hydrogen bond which calls C-H bond. The most commonly occurring elements in organic compounds are carbon hydrogen oxygen nitrogen sulphur and halogen elements.
The properties of a compound are largely determined by the functional group. The name chromatography comes from the Greek word chroma meaning colour and graphy for writing because the method was first used for the separation of coloured substances found. The boiling points of the alkanes gradually increase with the.
In fact there are many times more organic compounds known than all the other inorganic compounds discovered so far about 7 million organic compounds in total. Any of a class of organic compounds containing a carboxyl functional groupa carbon with a double bond to an oxygen and a single bond to another oxygen which is in turn bonded to a hydrogen. Difference Between Organic And Inorganic Compounds - Organic compound is a chemical compound of living things which contains Carbon and carbon atoms because of their relations with organisms.
In chemistry a ketone ˈ k iː t oʊ n is a functional group with the structure R 2 CO where R can be a variety of carbon-containing substituentsKetones contain a carbonyl group a carbon-oxygen double bond. The Chemistry of Ethers 1. The alkane that contains three carbon atoms is known as propane which has the formula C 3 H 8 and the following skeleton structure.
In organic chemistry the species that leaves the parent molecule following a substitution reaction. The four-carbon alkane is butane with the formula C 4 H 10. There is no direct method for the detection of oxygen.
56 Recognizing Common Organic Functional Groups. Organic chemistry was once thought to be confined to the study of substances produced as part of the natural processes of living organisms but as Friedrich Wohler discovered in the early 1800s organic compounds can be synthesized from minerals and other non-organic materials in the laboratory. The oldest artificially produced metal cluster may be calomel Hg 2 Cl 2 which was known in India already in the 12th century.
The number of known organic compounds is a quite large. This is a modern method that we can use for the separation of mixtures into its components purification of compounds and also test the purity of compounds. The elucidation of the structure of cluster compounds only became possible in the 20th century.
These reactions can occur in. The names formulas and physical properties for a variety of alkanes with the generic formula C n H 2n2 are given in the table below.

Benzene Definition Discovery Structure Properties Uses Britannica

Ch105 Chapter 9 Organic Compounds Of Oxygen Chemistry

10 Organic Chemistry Flashcards Quizlet
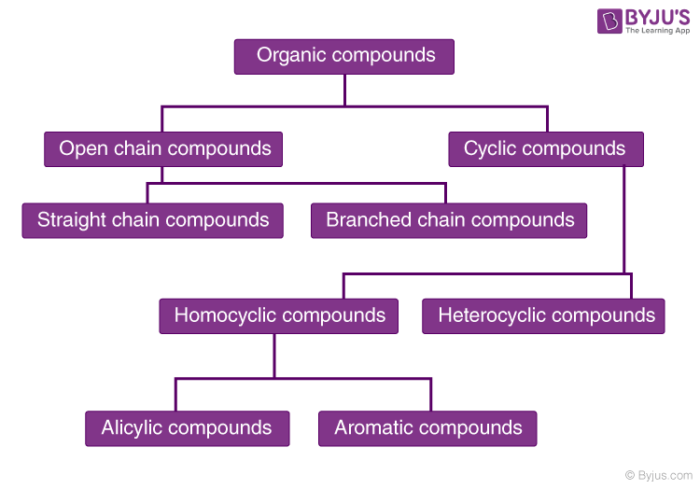
Organic Compounds Definition Examples Classification Of Organic Compounds

Organic Molecules Microbiology

Types Of Organic Reactions Explanation Examples Reactions Videos
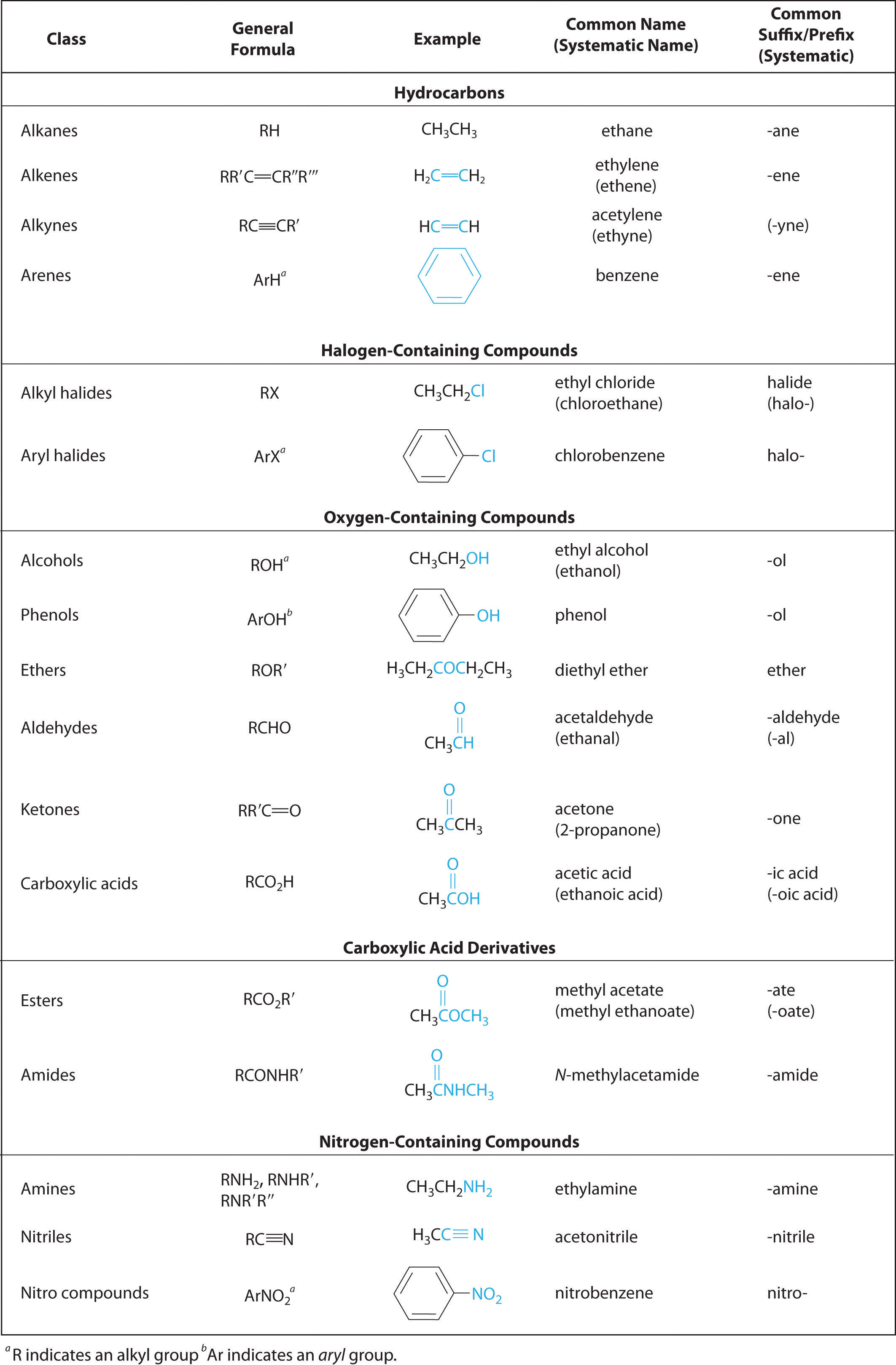
Functional Groups And Classes Of Organic Compounds

Different Functional Groups For Various Classes Of Organic Compounds In Download Scientific Diagram
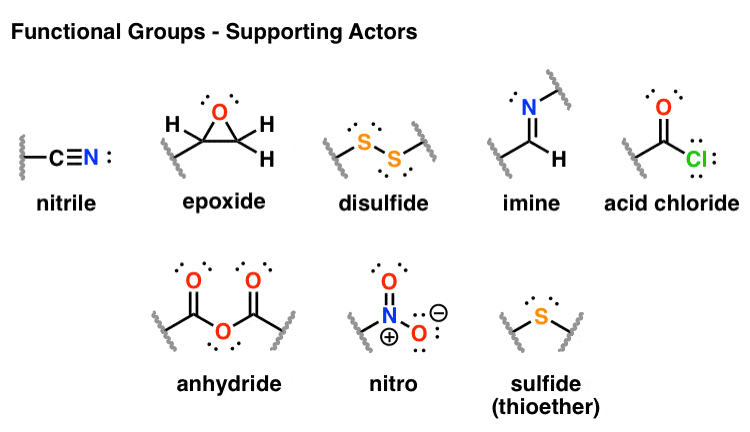
Functional Groups In Organic Chemistry

General Introduction To Organic Compounds Properties Uses With Videos
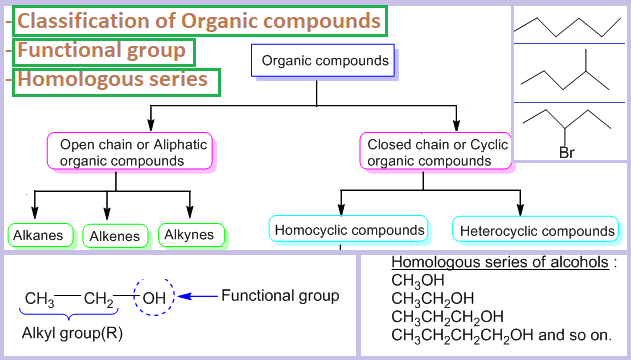
Organic Compounds Classification Functional Group And Homologous Series

Classes Of Organic Compounds Boundless Chemistry

Classification Of Organic Compounds Concepts Videos Solved Examples

Organic Compounds Classification Functional Group And Homologous Series
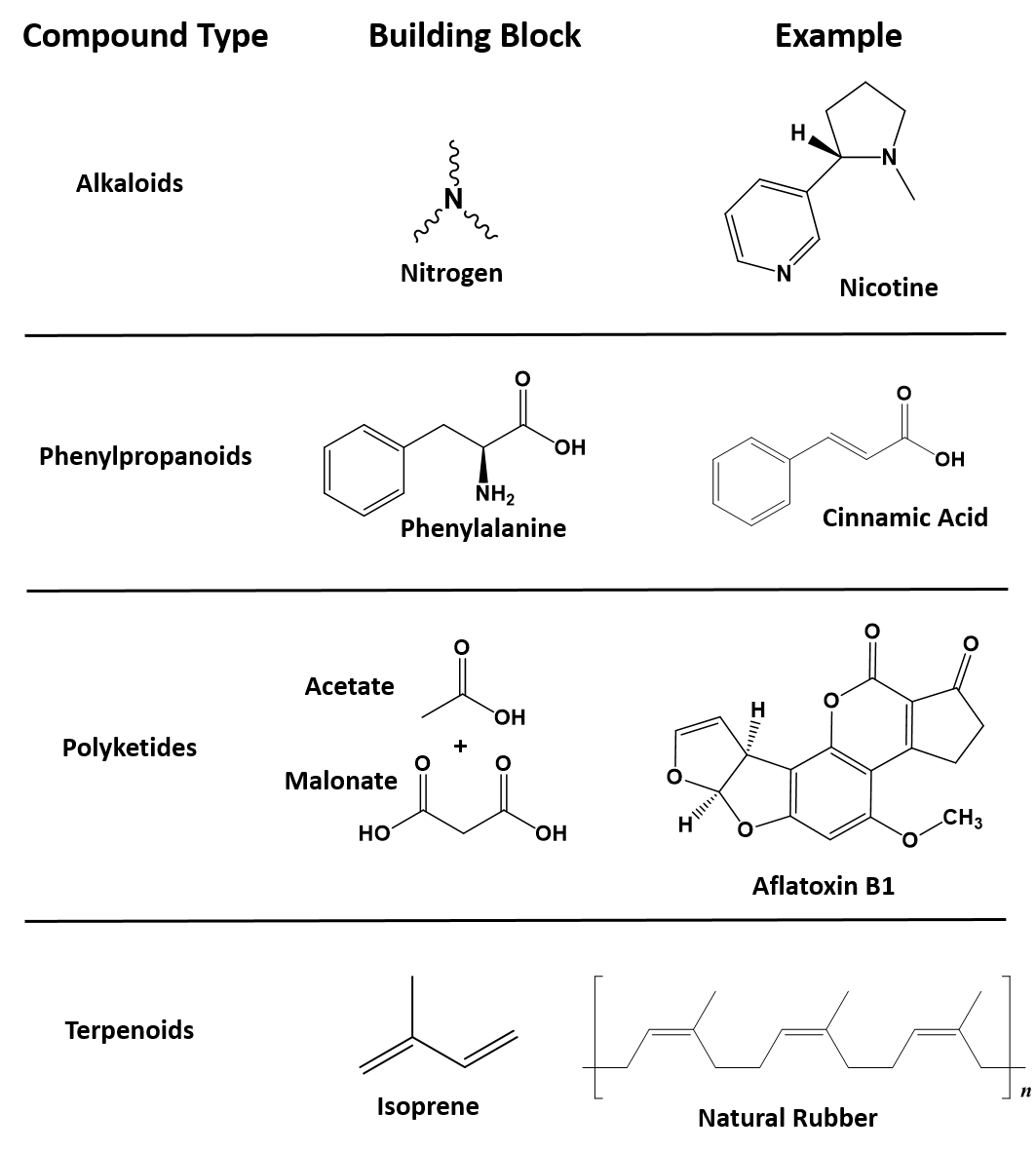
Ch105 Chapter 6 A Brief History Of Natural Products And Organic Chemistry Chemistry
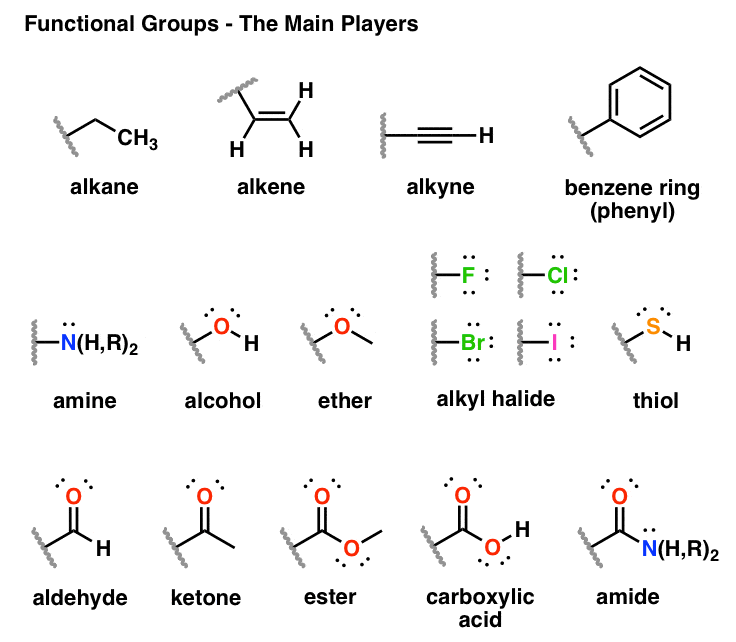
Functional Groups In Organic Chemistry

Functional Groups And Classes Of Organic Compounds